Hemodynamic Interfaces
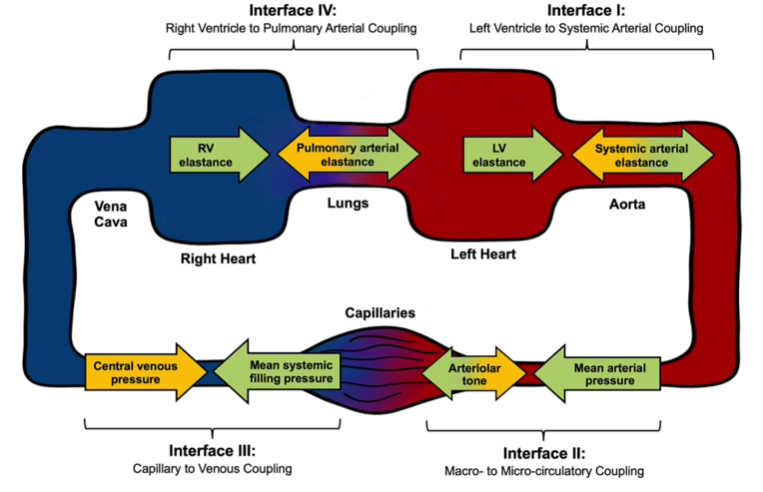
Systematic hemodynamic assessment through four fundamental interfaces: LV-Systemic Arterial, Macro-Microcirculatory, Capillary-Venous, and RV-Pulmonary Arterial coupling.
Interface ILV to Systemic Arterial Coupling
Interface IIMacro- to Micro-circulatory Coupling
Interface IIICapillary to Venous Coupling
Interface IVRV to Pulmonary Arterial Coupling
Hemodynamic Interfaces
Systematic hemodynamic assessment through four fundamental interfaces: LV-Systemic Arterial, Macro-Microcirculatory, Capillary-Venous, and RV-Pulmonary Arterial coupling.

Interface ILV to Systemic Arterial Coupling
Interface IIMacro- to Micro-circulatory Coupling
Interface IIICapillary to Venous Coupling
Interface IVRV to Pulmonary Arterial Coupling
Interpretation
Interface I: LV to Systemic Arterial Coupling - Insufficient data
Please enter hemodynamic variables to assess this interface.
Interface II: Macro- to Micro-circulatory Coupling - Insufficient data
Please enter hemodynamic variables to assess this interface.
Interface III: Capillary to Venous Coupling - Insufficient data
Please enter hemodynamic variables to assess this interface.
Interface IV: RV to Pulmonary Arterial Coupling - Insufficient data
Please enter hemodynamic variables to assess this interface.
Theory and Practice
The Need for a More Comprehensive Approach to Shock Assessment
Traditional shock frameworks—hypovolemic, distributive, cardiogenic, obstructive—are useful for teaching but overly simplistic for the complex physiology encountered at the bedside. Real patients rarely fit neatly into a single bucket, and management that focuses narrowly on mean arterial pressure (MAP) or stroke volume misses other critical contributors to organ failure.
The Hemodynamic Interfaces Paradigm proposed by Rola and colleagues reframes shock as the breakdown of coupling across four circulatory interfaces: LV–arterial, arterial–capillary, capillary–venular, and RV–pulmonary arterial. This framework highlights that shock can arise not only from inadequate forward flow but also from congestive injury and microcirculatory dysfunction.
The Concept of Coupling
The term coupling is borrowed from engineering, where it describes two systems working together in harmony to maximize efficiency with minimal wasted energy. This concept can be illustrated with the analogy of biking up a hill:
- Too low a gear → the cyclist pedals quickly but generates little forward motion (wasted effort, little output)
- Too high a gear → the cyclist struggles against resistance, leading to inefficiency
Optimal coupling lies in the middle, where effort and resistance are balanced for efficient forward motion.
Physiologically, the same principle applies where forward and backward pressures, driving forces, and resistances meet in the circulatory system. When these pressures are balanced, systems are coupled and energy is efficiently transmitted. When mismatched, they become uncoupled, and the circulation fails—whether from poor forward flow, excess congestion, or collapse of microcirculatory coherence.
Interface I: Left Ventricular to Systemic Arterial Coupling
At this interface, LV contractility is opposed by arterial elastance. Optimal ventriculo–arterial coupling ensures efficient energy transfer. Uncoupling arises when afterload rises excessively or when LV contractility falls.
Bedside indicators:
- Narrow or narrowing pulse pressure suggests a decline in stroke volume (typically >40 mmHg correlates with normal stroke volume)
- Reduced ejection fraction (EF) can reflect uncoupling, though interpretation is complicated by chronicity
- LVOT-VTI provides a real-time surrogate for stroke volume and can help identify acute uncoupling
- Corrected carotid flow time can serve as a surrogate for reduced LV function, though it is load-dependent
Formal calculation: Ventriculo–arterial coupling is expressed as Ea/Ees, where Ea (effective arterial elastance) ≈ (0.9 × SBP) / SV and Ees (end-systolic elastance) is estimated using Chen's single-beat method.
Interface II: Macro- to Microcirculatory Coupling
Here, changes in macrocirculatory variables (MAP, SV, DBP) must translate into effective microcirculatory perfusion:
- Hemodynamic coherence → manipulating MAP or cardiac output improves capillary flow
- Incoherence → microcirculatory flow remains impaired despite optimized macrohemodynamics
Bedside surrogates:
- Capillary refill time (CRT): validated as a rapid, evidence-based marker of coherence (normal <2 seconds)
- Skin mottling score: ranges from 0–5, associated with mortality and impaired perfusion
- Arterial–venous pCO₂ gap, central venous saturation, or NIRS-derived tissue oxygenation
Critical insight: When DBP falls below the critical closing pressure (Pcc), arterioles collapse, preventing flow into capillaries despite an apparently adequate MAP. Thus, "not all MAPs are created equal."
Early on in the resuscitation of shock patients are typically hemodynamically coherent, however this changes over time. This is due to the underlying disease process itself (for example, in sepsis, dysfunction and microthrombi formation). But can also result from iatrogenic causes like volume overload, where elevated interstitial pressure actually impairs capillary function. This may account for some of the observations that volume overload is an independent predictor of mortality for critically ill patients.
Interface III: Capillary to Venular Coupling
This interface highlights the venous side of circulation, where increased venous pressures can impede tissue outflow and impair organ perfusion as much as reduced MAP.
Assessment tools:
- Jugular venous pressure (exam or ultrasound) and CVP measurement
- Venous Doppler for abnormal waveforms (e.g., S < D, pulsatility, retrograde flow)
- VExUS score: integrates IVC, hepatic, portal, and intrarenal Doppler
- Renal Venous Stasis Index (RVSI): quantifies venous congestion in the kidney
Coupling fails when venous pressure rises and exerts excess tissue afterload. In this paradigm, venous pressure is preload for the RV but afterload for tissues—so rising CVP can directly reduce capillary and organ perfusion.
Interface IV: Right Ventricle to Pulmonary Artery Coupling
This mirrors Interface I, but for the RV and pulmonary circulation. RV contractility (Ees) must be matched to pulmonary arterial elastance (Ea).
Causes of uncoupling:
- Excessive pulmonary afterload (pulmonary hypertension, ARDS, PE)
- Intrinsic RV dysfunction or ischemia
Bedside surrogates:
- TAPSE/PASP ratio (<0.36 suggests uncoupling)
- RV S', Fractional Area Change (FAC), or RVOT Doppler morphology
- Elevated CVP and abnormal CVP waveforms
Uncoupling here can propagate backward, elevating CVP and worsening Interface III (venous congestion), while also impairing forward flow.
It is important to note that elevated PA pressures may be intrinsic to the pulmonary circulation itself or result from elevated left-sided pressure being transmitted retrograde. The pulmonary venous pressure directly contributes to the afterload of the RV, and thus interfaces 1 and 4 are closely related for patients.
Synthesis
Coupling theory emphasizes that shock physiology cannot be captured by MAP alone. Each circulatory interface represents a point where forward driving forces meet backward resistance, and efficiency is determined by balance.
Uncoupling at any interface can lead to shock—sometimes independently, sometimes sequentially in a cascade. At the bedside, the clinician's task is not to chase a single number but to deliberately interrogate each interface, identify where coupling is failing, and tailor therapy accordingly—whether by restoring preload, modifying afterload, augmenting contractility, or relieving venous congestion.
Contributors

Dr. Ross Prager
